This person is not a real SYMTUZA® patient.
Proven effective for people new to treatment
In 2 studies, SYMTUZA® was proven to be effective for people who were new to treatment. Some people received treatment within 14 days of diagnosis.
In the study focused on people who were new to treatment:
725 patients who were starting HIV treatment for the first time were split into two groups—one taking SYMTUZA® and one taking a different treatment.
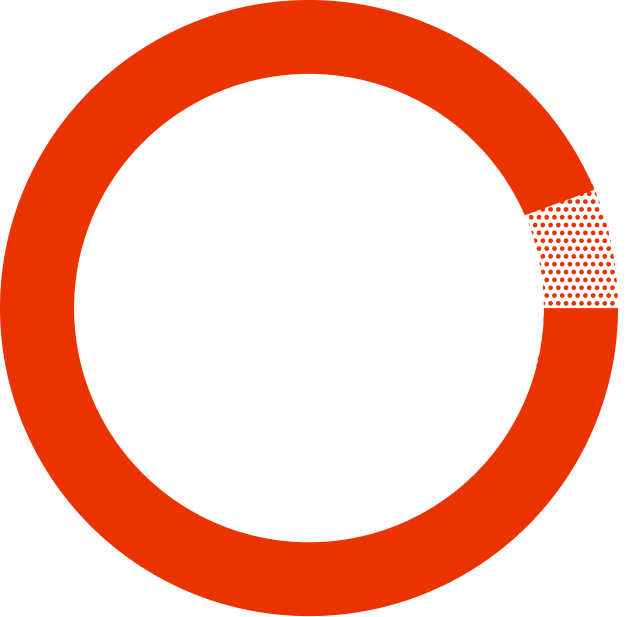
people who were treated with SYMTUZA® reached undetectable* after 48 weeks.
In the study focused on people who received treatment within 14 days after diagnosis:
92 /109 people
92 out of
109 people
![]()
![]()
reached undetectable* at 48 weeks after rapidly starting treatment with SYMTUZA®.
100%
![]()
![]()
of patients on SYMTUZA® were able to decrease their viral load to a low level (<200 copies/mL) after 48 weeks.†
97%
![]()
![]()
of people in this clinical trial were satisfied after rapidly starting treatment with SYMTUZA®.‡
*Undetectable is defined as the amount of HIV in the blood being below 50 copies/mL, meaning it cannot be measured by a lab test.
†Indicates observed analysis (this excludes patients who may have discontinued early or had missing data; n=96)
‡When asked “How satisfied are you with your present treatment?” in a standardized questionnaire, 97% (93 out of 96) reported that they were satisfied. Out of 109 patients, 96 of them completed a 10-question standardized questionnaire (HIVTSQs) based on a scale of 0-6 (0 = low favorability and 6 = high favorability). Satisfied was defined as reporting a score of 5 or 6. Between 87.8% and 99% of patients responded to individual questions with a score of 5 of 6. Mean total score for the questionnaire was 58 out of 60 at 48 weeks.
This person is not a real SYMTUZA® patient.
Proven effective for people undetectable and switching treatment
In a study, SYMTUZA® was shown to be effective and tolerable for people who were undetectable* and switched from a different treatment.†
This study had 1,141 patients, 763 of whom switched to SYMTUZA® and 378 of whom continued their current treatment.
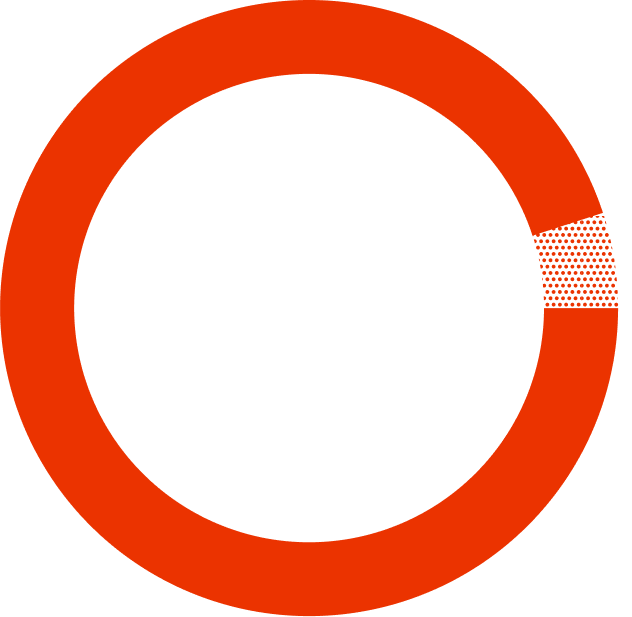
who were treated with SYMTUZA® stayed undetectable* after 48 weeks.
*Undetectable is defined as the amount of HIV in the blood being below 50 copies/mL, meaning it cannot be measured by a lab test.
†Patients were undetectable for at least 6 months.
This person is not a real SYMTUZA® patient.